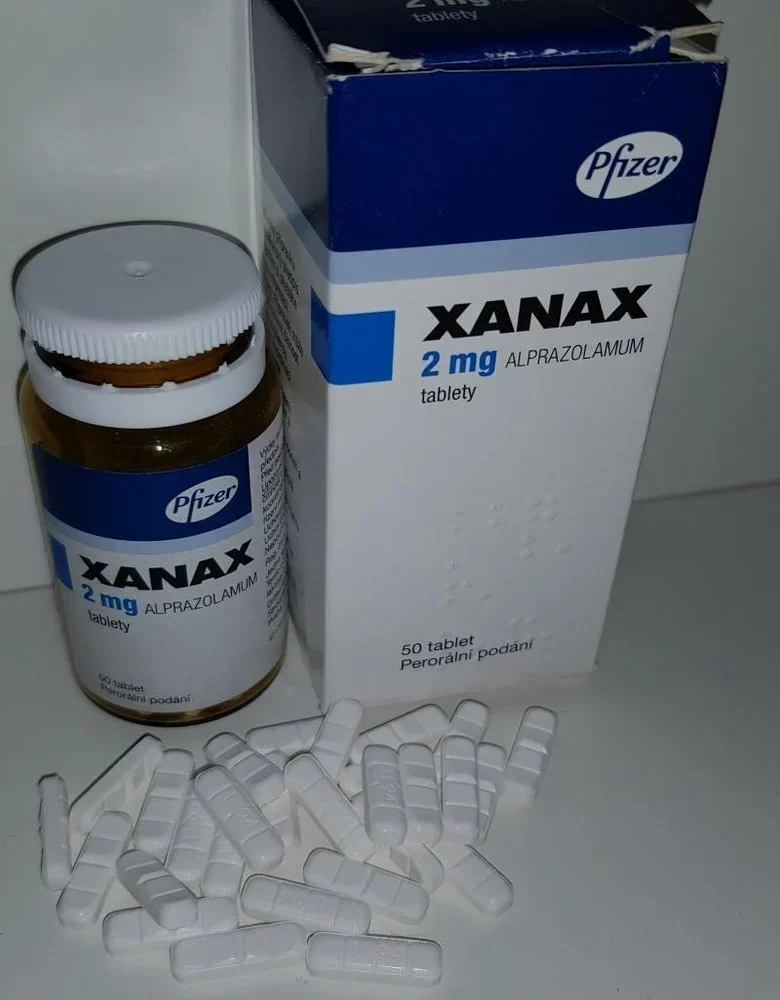
XANAX
- Generic Name: alprazolam
- Brand Name: Xanax
- Drug Class: , Anxiolytics, Benzodiazepine
Xanax 2mg (alprazolam) is a benzodiazepine used as an anti-anxiety medication prescribed to treat panic attacks and anxiety disorders. Xanax 2mg is available in generic form.
This product contain ALPRAZOLAM which is a triazolo ANALOG of the 1,4 benzodiazepine class of CENTRAL NERVOUS SYSTEM -active compounds.
The chemical name of alprazolam is 8-Chloro-1-methyl-6-phenyl-4H-s-triazolo [4,3-α] [1,4] benzodiazepine.
Alprazolam is a white crystalline powder, which is soluble in methanol or ethanol but which has no appreciable solubility in water at physiological pH.
Each Xanax 2mg Tablet, for oral administration, contains 0.25, 0.5, 1 or 2 mg of alprazolam.
XANAX Tablets, 2 mg, are multi-scored and may be divided
Dosage In Generalized Anxiety Disorder
The recommended starting oral dosage of XANAX for the acute treatment of patients with GAD is 0.25 mg to 0.5 mg administered three times daily. Depending upon the response, the dosage may be adjusted at intervals of every 3 to 4 days. The maximum recommended dosage is 4 mg daily (in divided doses). Xanax 2mg
Use the lowest possible effective dose and frequently assess the need for continued treatment [see WARNINGS AND PRECAUTIONS].
Dosage In Panic Disorder
The recommended starting oral dosage of XANAX for the treatment of PD is 0.5 mg three times daily. Depending on the response, the dosage may be increased at intervals of every 3 to 4 days in increments of no more than 1 mg per day.
Controlled trials of XANAX in the treatment of panic disorder included dosages in the range of 1 mg to 10 mg daily. The mean dosage was approximately 5 mg to 6 mg daily. Occasional patients required as much as 10 mg per day.
For patients receiving doses greater than 4 mg per day, periodic reassessment and consideration of dosage reduction is advised. In a controlled postmarketing dose-response study, patients treated with doses of XANAX greater than 4 mg per day for 3 months were able to taper to 50% of their total maintenance dose without apparent loss of clinical benefit.
The necessary duration of treatment for PD in patients responding to XANAX is unknown. After a period of extended freedom from panic attacks, a carefully supervised tapered discontinuation may be attempted, but there is evidence that this may often be difficult to accomplish without recurrence of symptoms and/or the manifestation of withdrawal phenomena [see Discontinuation Or Dosage Reduction Of XANAX].
Discontinuation Or Dosage Reduction Of Xanax 2mg
To reduce the risk of withdrawal reactions, use a gradual taper to discontinue XANAX or reduce the dosage. If a patient develops withdrawal reactions, consider pausing the taper or increasing the dosage to the previous tapered dosage level. Subsequently decrease the dosage more slowly [see WARNINGS AND PRECAUTIONS, Drug Abuse And Dependence].
Reduced the dosage by no more than 0.5 mg every 3 days. Some patients may benefit from an even more gradual discontinuation. Some patients may prove resistant to all discontinuation regimens.
In a controlled postmarketing discontinuation study of panic disorder patients which compared the recommended taper schedule with a slower taper schedule, no difference was observed between the groups in the proportion of patients who tapered to zero dose; however, the slower schedule was associated with a reduction in symptoms associated with a withdrawal syndrome.
Dosage Recommendations In Geriatric Patients
In geriatric patients, the recommended starting oral dosage of XANAX is 0.25 mg, given 2 or 3 times daily. This may be gradually increased if needed and tolerated. Geriatric patients may be especially sensitive to the effects of benzodiazepines. If adverse reactions occur at the recommended starting dosage, the dosage may be reduced [see Use In Specific Populationshttps://www.medicalnewstoday.com/articles/drugs-xanax, CLINICAL PHARMACOLOGY].
Dosage Recommendations In Patients With Hepatic Impairment
In patients with hepatic impairment, the recommended starting oral dosage of XANAX is 0.25 mg, given 2 or 3 times daily. This may be gradually increased if needed and tolerated. If adverse reactions occur at the recommended starting dose, the dosage may be reduced [see Use in Specific Populations, CLINICAL PHARMACOLOGY].
Dosage Modifications For Drug Interactions
Xanax 2mg should be reduced to half of the recommended dosage when a patient is started on ritonavir and XANAX together, or when ritonavir administered to a patient treated with XANAX. Increase the XANAX dosage to the target dose after 10 to 14 days of dosing ritonavir and XANAX together. It is not necessary to reduce ALPRAZOLAM dose in patients who have been taking ritonavir for more than 10 to 14 days.
XANAX is contraindicated with concomitant use of all strong CYP3A inhibitors, except ritonavir [see CONTRAINDICATIONS, WARNINGS AND PRECAUTIONS].
HOW SUPPLIED
Dosage Forms And Strengths
XANAX tablets are available as:
- 0.25 mg: white, oval, scored, imprinted “XANAX 0.25”
- 0.5 mg: peach, oval, scored, imprinted “XANAX 0.5”
- 1 mg: blue, oval, scored, imprinted “XANAX 1.0”
- 2 mg: white, oblong, multi-scored, imprinted “XANAX ” on one side and “2” on the reverse side
Be the first to review “ Xanax 2mg” Cancel reply
Related products
Uncategorized
Uncategorized
Uncategorized
Uncategorized






Reviews
There are no reviews yet.